By Todd Taylor
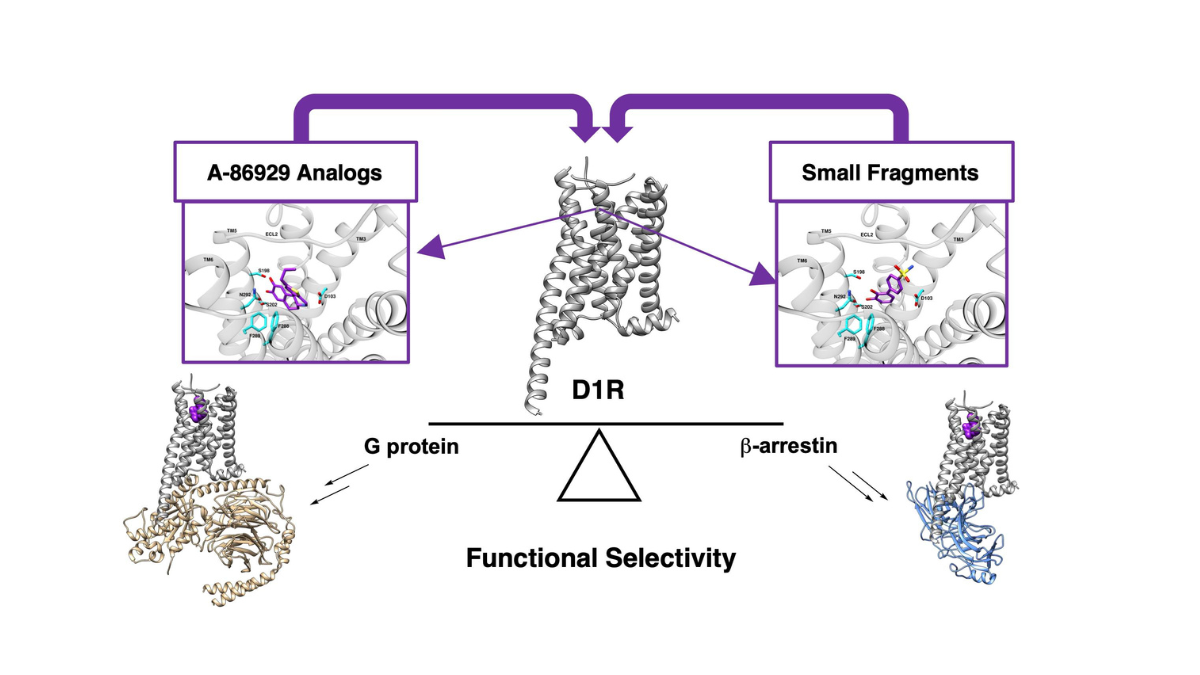
A new study by University of Florida neuroscientists provides insight into how drug molecules may bind to dopamine D1 receptors, interactions that could be key to developing improved therapies with fewer side effects for dopamine dysfunction.
Dopamine is a neurotransmitter that plays a role in many functions of the body such as movement, mood, learning and attention, and its dysfunction is implicated in several psychiatric and neurological disorders. Current dopamine receptor-based therapies are used to reduce motor deficits in Parkinson’s disease and as antipsychotic medications for schizophrenia, but efficacy may be limited to treating certain symptoms caused by dopamine dysfunction and may result in debilitating side effects.
The UF research team, led by Nikhil Urs, Ph.D. and Nicole Horenstein, Ph.D, used medicinal chemistry to generate small aryl fragments, fluorescent drug screening assays and computerized docking simulations to conduct their research, published in the journal ACS Chemical Neuroscience.
Urs said they found potential interactions within the receptor binding pocket, or a cavity within a protein, that could contribute to specific signaling at dopamine D1 receptors.
“This will provide a molecular map to design potential new therapies aimed at signaling biased ligands,” said Urs, an assistant professor of pharmacology and therapeutics.
The next step, he said, is to modify biased ligands, or small molecules or proteins that preferentially activate certain pathways, to increase their efficacy while maintaining their biased signaling properties.

