By Michelle Jaffee
A team of University of Florida neuroscientists and biomedical engineers report that a novel implantable device called MARTEENI showed promise in a rat-model study to potentially improve functionality of prosthetic devices designed to work with peripheral nerves in place of a lost limb.
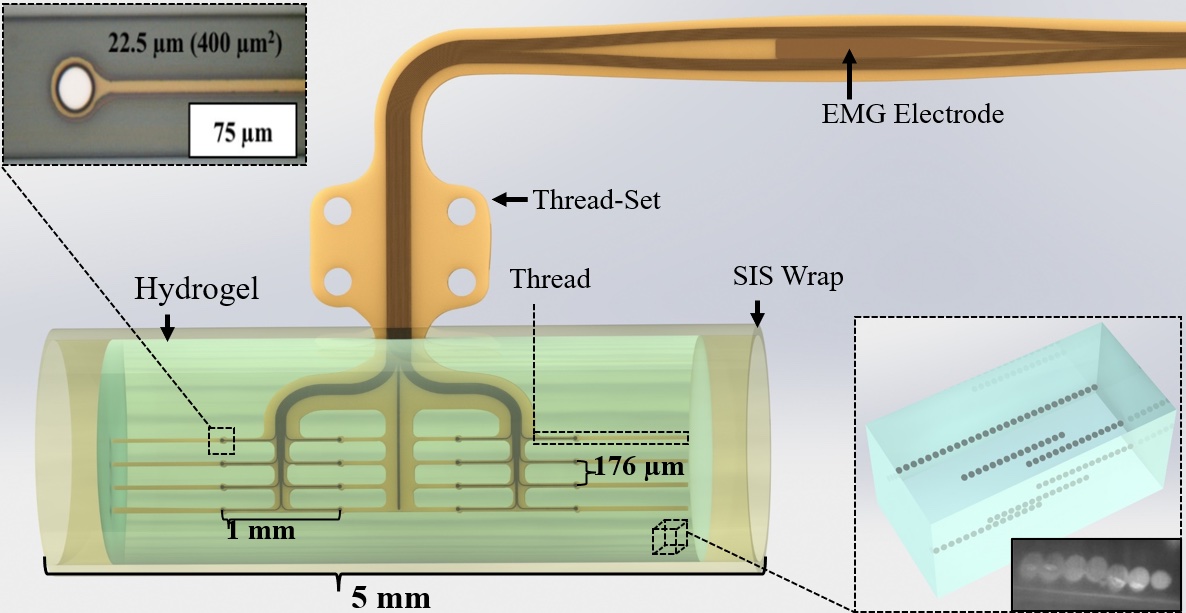
In the new study, published online in the Journal of Neural Engineering in August, the authors highlight the importance of high-bandwidth neural devices to advance the next generation of neural-enabled prosthetics and suggest that a promising approach is the MARTEENI device, which stands for Magnetically Aligned Regenerative Tissue-Engineered Electronic Nerve Interface.
The study is the first in vivo functional assessment of the MARTEENI device, which was designed and produced at UF in a multidisciplinary collaboration.
“This study builds upon previous clinical trials showing that neural-enabled prostheses have the potential to improve functionality and freedom of movement,” said Eric W. Atkinson, Ph.D., who as a doctoral student led the study with co-principal investigator Kevin Otto, Ph.D. “Our findings offer evidence that regenerative peripheral nerve interfaces could be a promising solution.”
The research team also included co-principal investigators Jack Judy, director of UF’s Nanoscience Institute for Medical & Engineering Technology; Christine Schmidt, Ph.D., chair of the J. Crayton Pruitt Family Department of Biomedical Engineering; and Carlos M. Rinaldi-Ramos, Ph.D., chair of chemical engineering.
The MARTEENI device is designed to utilize the natural tendency of the peripheral nervous system to regenerate. In the study, MARTEENI devices suspended within a microchannel-embedded, tissue-engineered hydrogel were implanted in the gaps of severed sciatic nerves in rats. Electrophysiology was recorded, and channel-isolated activity was observed in both acute and chronic experiments, the research team reported.
The findings show a high likelihood that observed electrophysiological activity recorded from implanted MARTEENIs originated from neural tissue, they said.
“MARTEENI represents a novel approach to interfacing with the peripheral nervous system,” Atkinson said. “Our results serve as a proof of concept that MARTEENI is capable of collecting channel-isolated electrophysiological activity in both acute and chronic settings.”

